
Blog Detail
The Fastest Way to get Your CPAKB Certification

Do you know what CPAKB is? Let's dig into what CPAKB is.
CPAKB stands for Certified Good Manufacture Practice of Medical Devices and In-Vitro Diagnostics. It is a guideline used in the process of manufacturing medical devices and quality control, aimed at ensuring that the produced healthcare products meet the specified requirements for their intended use.
The certification of CPAKB, known as Good Manufacturing Practice for Medical Devices, is an implementation of the Indonesian Health Law No. 36/2009, articles 96 and 107, regarding pharmaceutical preparations and medical devices. It is also in accordance with the Minister of Health Regulation No. 20/2017 on Good Manufacturing Practice for Medical Devices and Minister of Health Regulation No. 14/2021 on the Health Sector OSS (Online Single Submission), stating that the facilities for producing medical devices must have the necessary buildings and equipment according to the product requirements. The office and/or factory address should not be virtual, and they must comply with the standards outlined in the Minister's regulation regarding CPAKB.
The purpose of CPAKB is to ensure that the manufactured healthcare products, including medical devices and in-vitro diagnostic devices, meet the requirements, maintain their quality and safety, and fulfill their intended purposes. Manufacturing companies responsible for producing medical devices and in-vitro diagnostic devices are accountable for the quality, safety, and efficacy of their products. They must ensure that their products are made and produced in accordance with Good Manufacturing Practice, with no compromise in quality and performance during storage, use, and transportation processes.
The certification of CPAKB is given to manufacturing companies of medical devices and in-vitro diagnostics that have met the standard requirements in production, handling, and storage processes. This certification is issued by the Indonesian Ministry of Health. Every healthcare manufacturing business is obligated to comply with and adhere to the Minister of Health Regulation No. 20/2017 on Good Manufacturing Practice for Medical Devices. The manufacturing companies must possess a Manufacturing License for Medical Devices and In-Vitro Diagnostics (KLBI) with a designated number that corresponds to the Low, Medium, or High-Risk category. During the CPAKB certification process, the manufacturing companies are audited and evaluated by an auditing team, covering various aspects including:
Quality Management System - ISO 13485:2016
Management Responsibility
Human Resource Management
Product RealizationMeasurement, Analysis, and Improvement
The auditing team evaluates the quality management system of the manufacturing company, including its buildings and facilities, hygiene and pest control, raw material warehouse (receiving, storage, and shipping areas), production areas/lines, residue areas, quality control areas, packaging areas, and finished goods. Upon successfully passing the evaluation process, the manufacturing company of medical devices and in-vitro diagnostics will be granted the CPAKB certificate by the Ministry of Health through the Directorate General of Pharmaceutical and Medical Devices - Jo.
Directorate of Medical Device Supervision, indicating that the manufacturing company has met the standards set by the Ministry of Health. This certification instills confidence in consumers that the healthcare products produced by the company have undergone appropriate and safe processes. The data on the distribution of healthcare manufacturing companies in Indonesia
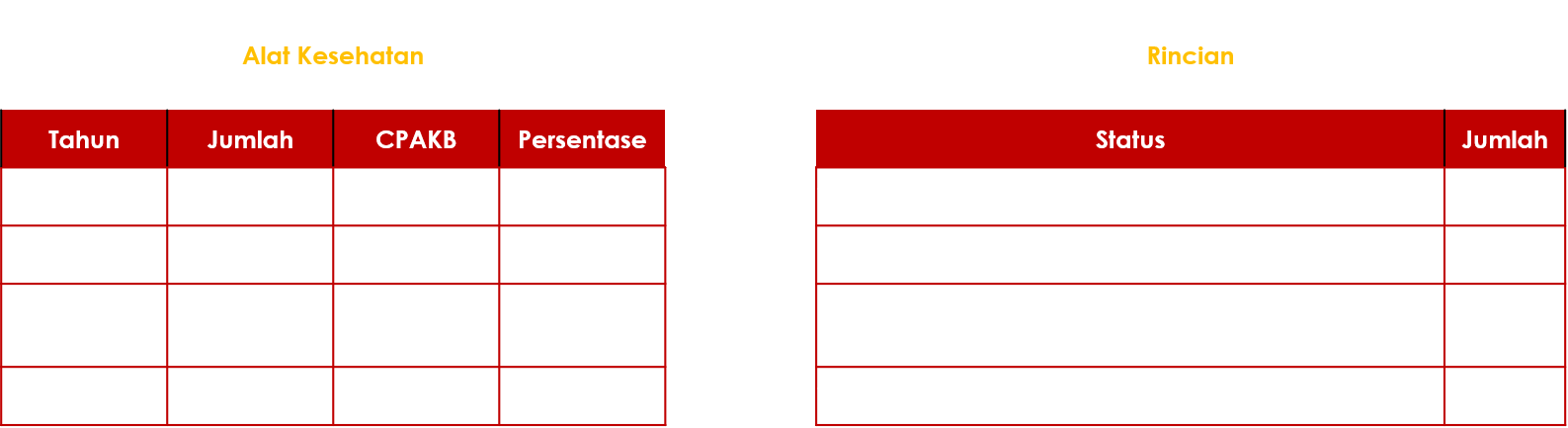
Source: Ditwaskes (Directorate of Health Facility Oversight).
In the context of the healthcare manufacturing industry in Indonesia, if you are a manufacturer of medical devices and in-vitro diagnostics, obtaining the CPAKB certification is mandatory and essential. It ensures the quality and safety of your products and the sustainability of your business.
Currently, until December 31, 2023, for registering domestically produced medical devices (AKD registration), manufacturers are required to commit to the ongoing CPAKB process. Starting from January 1, 2024, the Ministry of Health will enforce the law for every registration of medical devices and in-vitro diagnostics produced domestically (AKD registration) to be accompanied by the CPAKB certificate.
This measure aims to improve the quality of the healthcare industry for medical devices and in-vitro diagnostics in Indonesia, thereby achieving a higher level of safety in providing healthcare services to the public.
PT INSPIRY INDONESIA KONSULTAN is here for you, as a reliable partner, providing assistance in obtaining the CDAKB certification for manufacturers of in-vitro diagnostic medical devices. The assistance services include:
Preparation of legal documents
Preparation of 48-50 Standard Operating Procedures and over 100 forms
Work assistance socialization
Internal Audit socialization
Internal Audit assistance
Open-Closing Corrective and Preventive Actions (CAPA)
Submitting to OSS
External Audit
Open-Closing CAPA for external Audit
Issuance of CPAKB certificate
With our experience, dedication, and commitment, we are ready to help your company achieve the CPAKB certification. Together, we can strengthen the medical device industry and contribute to the economic growth of Indonesia.
