
Blog Detail
INSPIRY INDONESIA at CMEF Shanghai 2025: Regulatory Strategy, Global Partnerships in the Heart of Asia’s MedTech Revolution

CMEF Shanghai 2025: Where Global MedTech Finds Its Pulse
CMEF 2025 may be over, but its impact continues to resonate.
From April 8–11, 2025, the National Exhibition and Convention Center (NECC) Shanghai became the epicenter of global medical innovation—hosting over 5,000 exhibitors and visitors from more than 150 countries.
Tech giants such as GE Healthcare, United Imaging, and Neusoft unveiled next-generation solutions like the SIGNA™ Hero MRI, uAI NEXUS adaptive agents, and ultra-HD CT scanners.
But beyond the technology, CMEF 2025 was about ideas, debates, and partnerships—amplified by side events like ICMD and CECN.
At Booth H5.2J06, right beside the vibrant India Pavilion, INSPIRY INDONESIA stood out—not just as an exhibitor, but as a hub of cross-border regulatory expertise and market access.
This corner of the exhibition embodied a new MedTech Silk Road, connecting China, ASEAN, and the Middle East through collaboration, compliance, and shared healthcare ambitions.
INSPIRY INDONESIA: The Only Indonesian Voice in CMEF’s Global Dialogue
Among the sea of MedTech giants, INSPIRY INDONESIA carried the red-and-white flag with purpose—the only Indonesian consulting firm bridging global innovation with Southeast Asian healthcare realities.
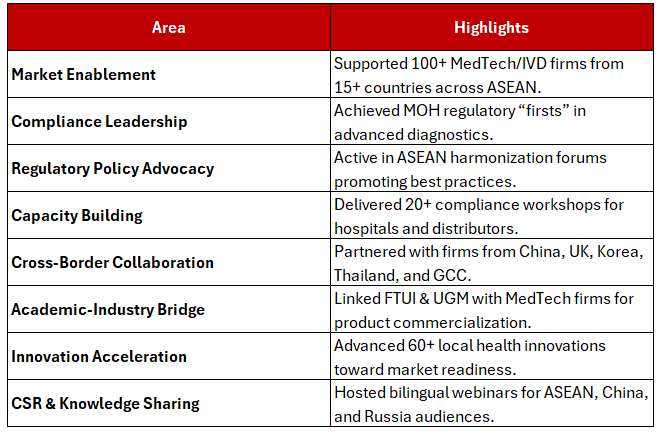
More than an exhibitor, INSPIRY acted as a live intelligence hub. Industry players from China, Japan, and Korea came not for brochures, but for clarity—how to navigate Indonesia’s regulatory and operational complexities.
Through multilingual briefings and strategy sessions, INSPIRY INDONESIA provided what the global MedTech community needed most: a roadmap to succeed in Indonesia’s high-potential market.
Key Expertise Showcased at CMEF 2025:
Regulatory pathway planning & submissions (MOH, BPOM, AMDD–ASEAN–China harmonization)
Product testing, calibration, and certification
Strategic market entry & distributor matchmaking
Import, distribution, and after-sales compliance
Healthcare infrastructure & digital transformation consulting
Post-market surveillance & compliance risk mitigation
CMEF visitors widely referred to the INSPIRY booth as a “must-visit” for anyone serious about expanding into Southeast Asia’s fast-growing healthcare sector.
MedTech and IVD Interactions: Frontline Perspectives
CMEF 2025 also spotlighted the surge of MedTech and IVD innovators from Japan, India, Korea, and beyond. With global supply chains shifting and Asia’s healthcare market booming, many sought pathways into Indonesia, a country at the crossroads of reform and opportunity.
Why Indonesia?
Compelling Market Size: Projected at US$2.26 billion in 2025, growing ~9% annually—among ASEAN’s fastest.
Universal Healthcare Access: Jaminan Kesehatan Nasional (JKN) now covers 98% of the population, fueling demand for diagnostics, imaging, consumables, and telemedicine.
Regulatory Shifts: Policies like TKDN (Domestic Component Level) spur local innovation and strategic foreign partnerships, though compliance remains complex.
Digital Transformation: Rapid growth in EHRs, remote monitoring, and IVD drives new opportunities for connected MedTech solutions.
At CMEF, INSPIRY INDONESIA became a translator, navigator, and market maker—helping innovators align compliance and strategy for real market results.
Who Is INSPIRY INDONESIA?
Founded in 2019, INSPIRY INDONESIA is the nation’s first and only consulting firm that integrates regulatory compliance, market enablement, and operational support for MedTech, IVD, and healthcare firms expanding across Indonesia and Southeast Asia.
Core Capabilities:
1. End-to-End Regulatory Strategy
Expertise in MOH, BPOM, and provincial compliance for all medical device classes (including digital health & high-risk items)
Regulatory mapping & technical dossier bundling
One-gate licensing & IOR advisory (License Holder strategy)
Blueprint facility design & QMS integration
Audit simulation, TKDN acceleration, and BMP scoring
2. Product Testing, Calibration & Certification
Guidance through pre-market testing via SNI ISO/IEC 17025:2017 labs
Clinical validation and local trial coordination
Post-market calibration (via BPAFK)
ISO 13485 & 9001 certification support
3. Market Entry Operations
Distributor vetting & e-Catalogue integration
Post-market surveillance & government procurement strategy
4. ASEAN Harmonization (AMDD)
Participation in ASEAN regulatory alignment and harmonization forums
5. Capacity Building
Training, recruitment, and knowledge transfer for healthcare and MedTech professionals
People Behind the Movement
At the heart of INSPIRY’s success is its people.
We proudly acknowledge the leadership of Apt. Syifa Amirta, Sani S.Farm (Director of Partnership & International Relations), Lukmanul Hakim (Director of Commercials), and Shyanel Lee (Head of Marketing)—along with their dynamic team.
Together, they connect entrepreneurs to regulators, MNCs to SMEs, and innovation to implementation. Under their guidance, INSPIRY INDONESIA isn’t just on the global map—it’s helping redraw it.
Why INSPIRY INDONESIA at CMEF Matters
INSPIRY INDONESIA’s participation at CMEF 2025 symbolizes a shift: Southeast Asia is no longer a peripheral market—it’s a center of innovation, compliance, and MedTech growth.
Through its partnership-driven, results-oriented approach, INSPIRY catalyzed new collaborations, accelerated regulatory success, and strengthened regional ecosystems.
For global innovators seeking to enter Indonesia and ASEAN, one message is clear:
👉 INSPIRY INDONESIA is not just your gateway—it’s your strategic partner shaping the future of health.
Partner Appreciation: Global Synergy in the Asia–MENA MedTech Ecosystem
The vibrancy of INSPIRY’s booth was fueled by cross-border partners including Legend Board (China), RegTrac (UK), SMG (Korea), Siam Trade (Thailand), and Aviis Healthcare (GCC/MENA).
Their collaboration enriched the firm’s global reach and strengthened regional MedTech connectivity.
INSPIRY INDONESIA extends its gratitude to all partners for making the CMEF 2025 presence a true crossroads of Asia–MENA MedTech ambition.
Ready to Accelerate Your ASEAN Healthcare Journey?
📩 Contact INSPIRY INDONESIA – your trusted partner for regulatory compliance, strategic operations, and sustainable MedTech growth across Indonesia and Southeast Asia.
Sources:
6W Research: Indonesia Medical Devices Market 2020–2026
#InspiryCMEF2025 #AsiaHealthcare #IndonesiaMedTech #GrowthHacking #RegulatoryCompliance #SEAMarketEntry #HealthcareInnovation #InspiryIndonesia #CMEFShanghai #GlobalMedTechASEAN
