
Blog Detail
Indonesia’s Voice in Global Healthcare Regulation at CIMDR & DIA China 2025

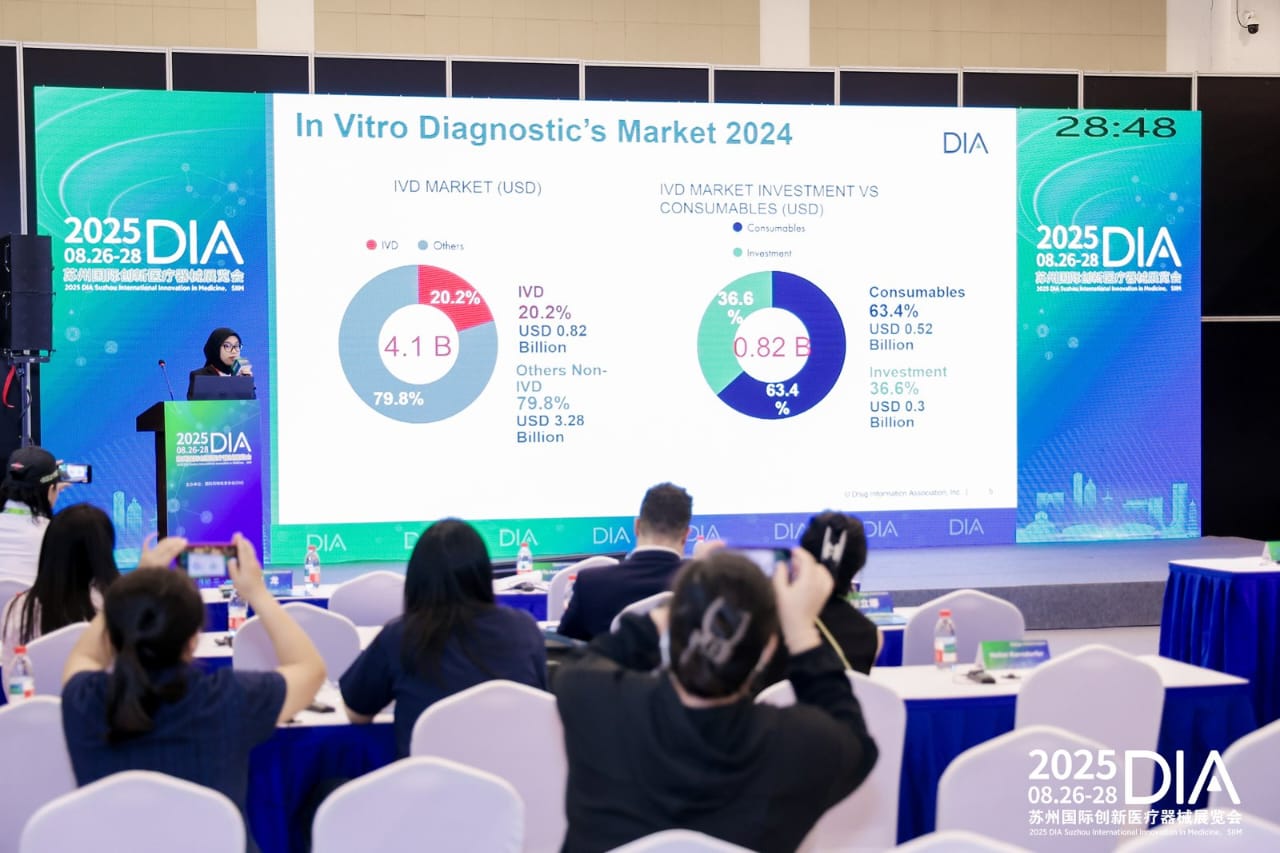
As Asia’s healthcare sector accelerates toward greater innovation and integration, the demand for strategic collaboration, regulatory expertise, and visionary leadership has never been stronger.
At the forefront of this transformation stands INSPIRY INDONESIA KONSULTAN—a leading consultancy firm specializing in regulatory compliance, operational strategy, and market enablement for the medical device industry.
Indonesia’s Presence on the Global Regulatory Stage
Inspiry’s recent participation in two of the region’s most prestigious international forums—the China International Medical Device Regulatory Forum (CIMDR) and the DIA China Annual Meeting in Suzhou—underscored its growing influence in shaping global healthcare dialogue and advancing regulatory harmonization.
Representing Inspiry on the global stage was Apt. Syifa Amrita Sani, S.Farm, Director of Partnership and International Relations.
Her role was not merely symbolic—it was strategic.
Through keynote insights and panel discussions, Syifa shared perspectives on Indonesia’s evolving regulatory landscape, offering practical guidance for multinational stakeholders navigating market entry and compliance pathways.
Her contributions helped bridge the gap between global regulatory frameworks and local implementation, reaffirming Inspiry’s position as a connector between international manufacturers and regional health authorities.
CIMDR & DIA Suzhou 2025: A Hub for Regulatory Innovation
Held on August 26–28, 2025 at the Jinji Lake International Conference Center, Suzhou, the 15th CIMDR Forum—often dubbed “The Davos of China’s Medical Device Regulation”—brought together top regulatory authorities, including the National Medical Products Administration (NMPA), alongside scientists and global industry leaders.
Key Themes Included:
Regulatory innovation and lifecycle management
International harmonization of standards
AI and digital health regulation
Post-market surveillance and clinical evaluation pathways
Complementing CIMDR, the DIA China Annual Meeting explored patient safety, quality systems, and cross-border compliance.
Inspiry’s active participation highlighted its expertise in:
Interpreting complex regulatory frameworks across Indonesia and China
Supporting global companies in market access and certification
Facilitating dialogue between Indonesian stakeholders and international regulators
Driving Strategic Collaboration Across Borders
During breakout sessions and roundtables, Syifa shared best practices for IVD registration, updates on Indonesia’s regulatory evolution, and insights into ASEAN-wide harmonization efforts.
These discussions elevated Indonesia’s visibility within the global regulatory community—positioning Inspiry as a key enabler of compliant expansion for Chinese and international manufacturers entering Southeast Asia.
Exploring Innovation at Medical Fair China & REHACARE 2025

Inspiry also attended Medical Fair China and REHACARE 2025, held from August 21–23, 2025, at the Suzhou International Expo Center.
These exhibitions showcased cutting-edge technologies in rehabilitation, assistive devices, and medical equipment.
A special note of appreciation goes to Legend Board Medical Group, Inspiry’s strategic partner, for their warm hospitality and collaborative engagement throughout the event.
Inspiry’s Global Impact: At a Glance

Bridging Borders, Empowering Innovation
INSPIRY INDONESIA KONSULTAN continues to champion Indonesia’s presence on the global healthcare stage.
Through consistent participation in international conferences and exhibitions, Inspiry not only strengthens its network but also empowers clients to thrive amid complex regulatory environments.
As the healthcare landscape evolves, Inspiry remains committed to delivering strategic insight, regulatory clarity, and market success—bridging borders and transforming possibilities.
Stay tuned as Inspiry continues shaping the future of healthcare regulation—one forum, one partnership, and one breakthrough at a time.
Ready to expand your healthcare footprint in Indonesia and ASEAN?
Partner with INSPIRY INDONESIA KONSULTAN to navigate regulatory complexity, unlock market potential, and achieve sustainable growth across Southeast Asia.
Sources:
#InspiryIndonesiaKonsultan #CIMDR2025 #DIAChina2025 #MedicalDeviceRegulation #ASEANHealthcare #IndonesiaHealthcare #IVDCompliance #RegulatoryAffairsAsia #HealthcareConsulting #MarketAccessASEAN #MedicalDeviceIndonesia #SyifaAmritaSani #GlobalToLocalHealthcare #SuzhouHealthcareEvents #HealthcareInnovation #RegulatoryCompliance #MedicalDevices #HealthTech #GlobalHealth #DigitalHealth #LifeSciences #HealthcareLeadership #PharmaRegulation #InternationalCollaboration #HealthPolicy #MedTechAsia #HealthcareStrategy #EmergingMarkets #HealthIndustryTrends
